The Challenge
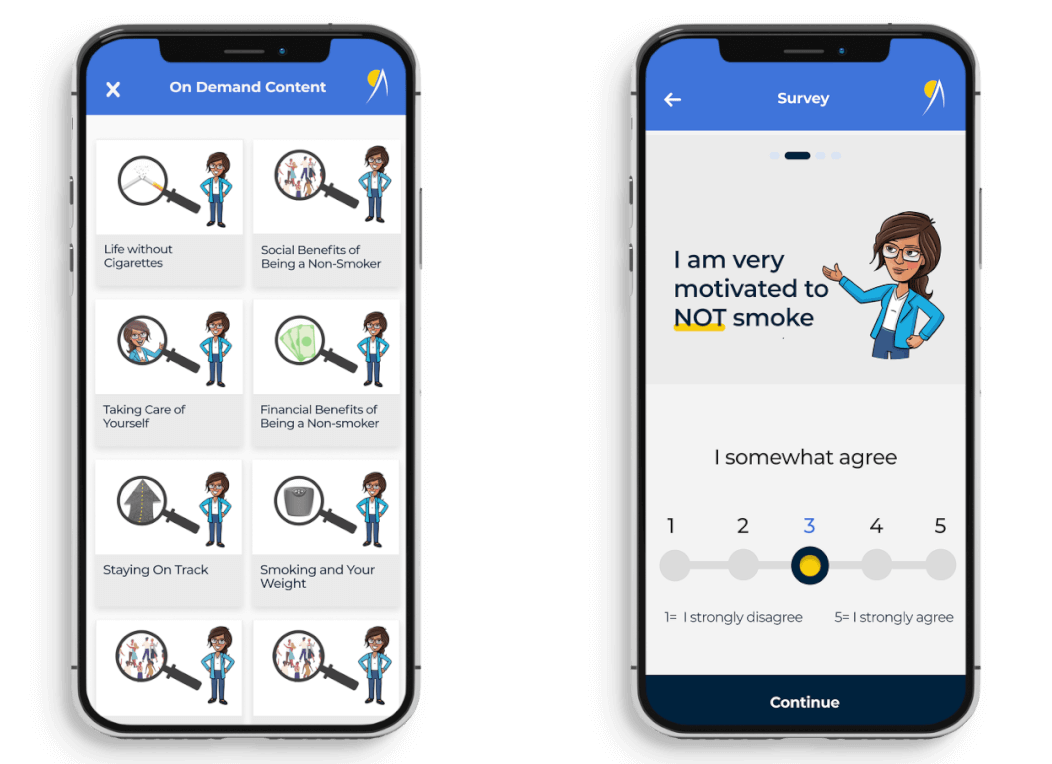
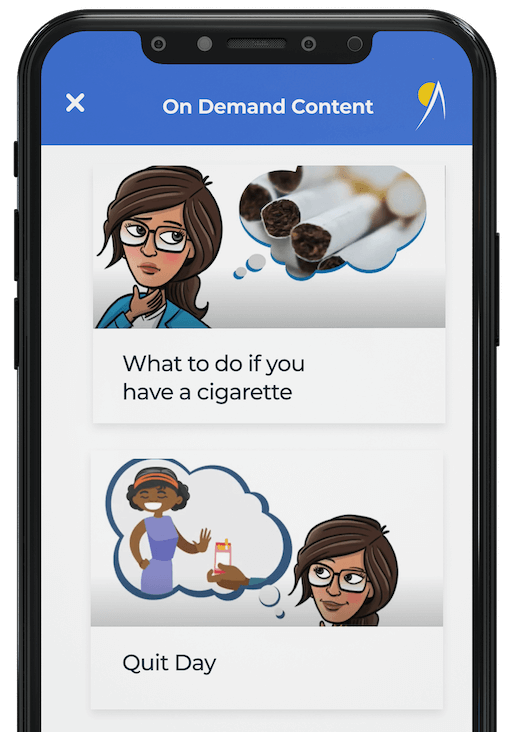
Moffitt Cancer Center is a National Cancer Institute (NCI) Designated Comprehensive Cancer Center and one of the leading cancer centers in the United States. Moffitt received two R01 grants from the NCI in 2019 to evaluate mobile smoking cessation interventions targeted to special populations (food pantry clients and people with HIV), and they turned to Dogtown Media to develop the mobile apps. The app-based interventions in both trials comprise a twelve-week treatment period, where participants are asked about their smoking status, motivation, confidence and stress level via an in-app survey. Based on their responses, participants are delivered customized treatment videos. Participants are also able to access personalized, on-demand content for a twenty-six-week period.
The Process
- User research, analysis and design discovery
- User flow diagrams using wireframes
- High fidelity graphics with interactive prototype
- Front-end development for iOS and Android
- Backend systems for storing user data and uploading content
The Solution
A streamlined app that can be easily navigated for users to complete surveys designed to personalize treatment content. Consisting of 12 weeks of structured, video-based treatment sessions and 26 weeks of On-Demand Treatment Content, these applications deliver a weekly in-app survey that assesses smoking status, motivation, self-efficacy, and stress. Participants are supplied with daily text content for the first 12 weeks, along with push notifications that remind them to stay engaged with the program as they strive to quit smoking and stay abstinent.
Both mobile apps include the following:
- Integration with a REDCap clinical database for storing all participant data
- Building the backend coding logic to determine which video combinations should be delivered to which participants during the twelve-week treatment periods.
- Creating an API that reliably and securely connects the app to the backend database.
- Personalized notifications sent to participants to encourage them to complete weekly surveys.
- Support for intervention delivery in English and Spanish.

The Result